1599 Geneva Bible (GNV)
The Geneva Bible: A Cornerstone of English Protestantism A Testament to Reform The 1599 Geneva Bible... Read More
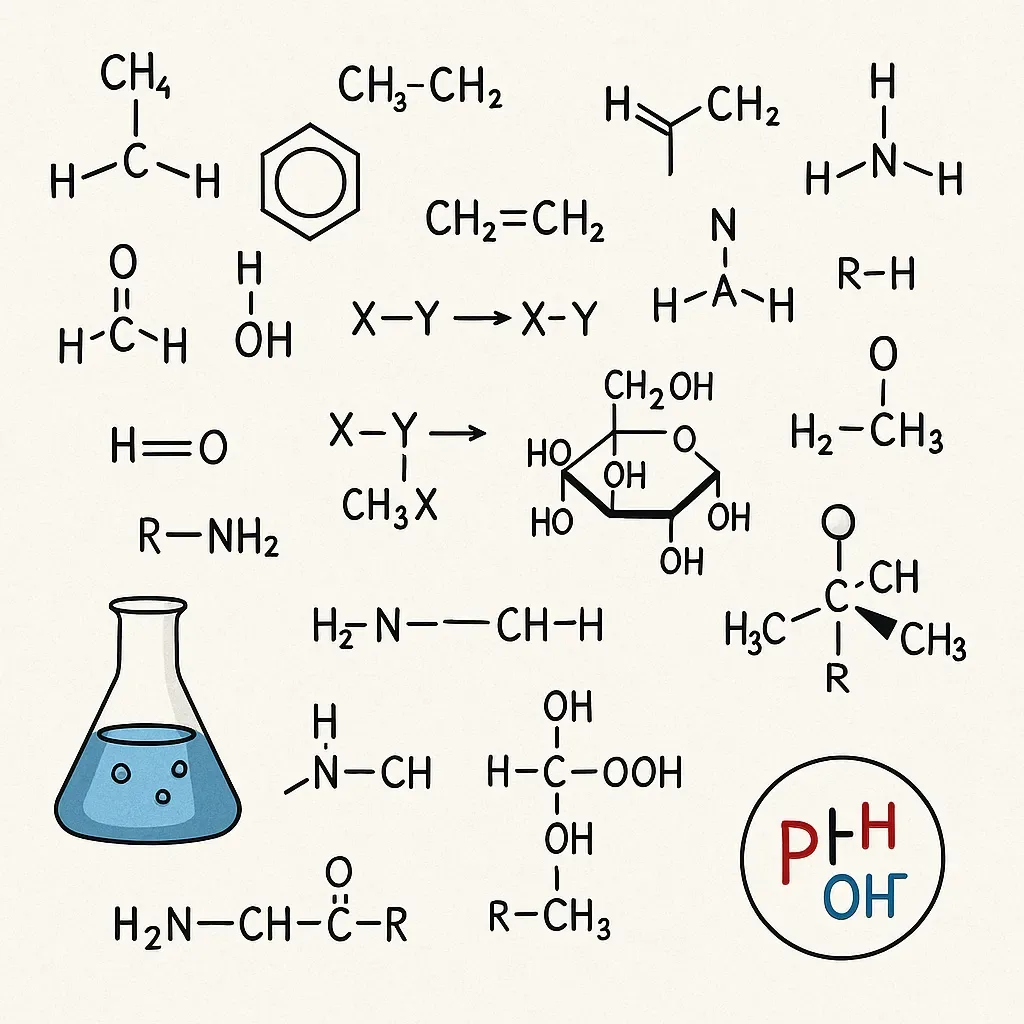
Organic chemistry is a vital sub-discipline of chemistry that deals with the structure, properties, composition, reactions, and synthesis of carbon-containing compounds. These compounds include not only hydrocarbons but also compounds with any number of other elements, including hydrogen, nitrogen, oxygen, halogens, phosphorus, silicon, and sulfur. The importance of organic chemistry extends across a vast range of scientific and industrial fields, from pharmaceuticals to agriculture, materials science, and even biochemistry.
Historically, organic chemistry was once thought to involve only compounds produced by living organisms. This notion changed dramatically with the synthesis of urea by Friedrich Wöhler in 1828, from an inorganic compound. His work broke the boundary between organic and inorganic chemistry, showing that organic compounds could be synthesized artificially. This laid the foundation for the modern field of organic chemistry.
Carbon's unique ability to form four covalent bonds and create stable chains and rings is the cornerstone of organic chemistry. These bonds can be single, double, or triple, leading to a wide variety of molecular geometries and complexities. Hybridization, such as sp³, sp², and sp, helps explain the bonding and shapes of organic molecules.
Functional groups are specific groupings of atoms within molecules that have their own characteristic properties. Common functional groups include hydroxyl (-OH), carbonyl (>C=O), carboxyl (-COOH), amino (-NH₂), and many more. These groups determine the reactivity and interactions of the molecules.
Organic compounds can exist in various forms known as isomers—molecules with the same molecular formula but different structures or spatial arrangements. Structural isomers differ in connectivity, while stereoisomers differ in spatial orientation. Chirality, a form of stereoisomerism, plays a significant role in the biological activity of molecules.
Organic reactions are categorized into several types:
These reactions are essential for building complex molecules, including drugs, plastics, dyes, and more.
One of the most crucial and often overlooked aspects of organic chemistry is the role of pH. Understanding pH in organic chemistry is essential for grasping how reactions occur and how compounds behave in different environments.
The term pH organic chemistry refers to the acidity or basicity of a solution and how it affects organic molecules. The pH can influence reaction rates, solubility, and even the structural stability of organic compounds. For instance, certain functional groups may become protonated or deprotonated depending on the pH, which can drastically change their reactivity.
In biological systems, the concept of ph in ochem is particularly important. Enzymes, which are organic catalysts, often function optimally within narrow pH ranges. A slight shift can render them inactive or less efficient. This also applies to pharmaceutical chemistry, where drug efficacy can depend on the ph in organic chemistry of the target environment, such as the stomach or bloodstream.
But what is pH in organic chemistry at its core? It’s a logarithmic scale that measures hydrogen ion concentration, and in organic chemistry, it can determine whether a compound acts as an acid or base. The Brønsted–Lowry theory and Lewis acid-base theory both hinge upon pH dynamics.
Moreover, for students and professionals alike, asking what does pH stand for in organic chemistry is not merely academic curiosity—it’s foundational knowledge. Whether one is working with buffer systems, analyzing reaction mechanisms, or designing synthesis routes, understanding pH is imperative.
Organic chemistry is ubiquitous in daily life and scientific innovation:
Today, organic chemistry is advancing through green chemistry initiatives, which aim to design products and processes that reduce or eliminate hazardous substances. The use of bio-based feedstocks, catalytic systems, and environmentally friendly solvents are becoming standard.
Computational chemistry and machine learning are also making headway in predicting reaction outcomes, optimizing synthesis pathways, and modeling complex organic systems.
Organic chemistry is the key to understanding life at the molecular level and driving innovation across industries. From the basic principles of carbon bonding to complex synthetic strategies, it offers powerful tools for solving real-world problems. And among its many foundational aspects, the role of ph ochem stands out as critical—affecting everything from drug design to metabolic processes.
As the field continues to evolve, so too will our capacity to manipulate organic molecules for the benefit of humanity and the planet.
The Geneva Bible: A Cornerstone of English Protestantism A Testament to Reform The 1599 Geneva Bible... Read More
The 21st Century King James Version (KJ21): A Modern Approach to a Classic Text A Balancing Act The ... Read More
The American Standard Version (ASV): A Cornerstone of Modern English Bibles A Product of Scholarly R... Read More
The Amplified Bible (AMP): A Rich and Comprehensive Translation The Amplified Bible (AMP) stands out... Read More
The Amplified Bible, Classic Edition (AMPC): A Timeless Treasure The Amplified Bible, Classic Editio... Read More
The Authorized (King James) Version (AKJV): A Timeless Classic The Authorized King James Version (AK... Read More
The BRG Bible: A Colorful Approach to Scripture A Unique Visual Experience The BRG Bible, an acronym... Read More
The Christian Standard Bible (CSB): A Balance of Accuracy and Readability The Christian Standard Bib... Read More
The Common English Bible (CEB): A Translation for Everyone The Common English Bible (CEB) is a conte... Read More
The Complete Jewish Bible (CJB): A Jewish Perspective on Scripture The Complete Jewish Bible (CJB) i... Read More
The Contemporary English Version (CEV): A Bible for Everyone The Contemporary English Version (CEV),... Read More
The Darby Translation: A Literal Approach to Scripture The Darby Translation, often referred to as t... Read More
The Disciples' Literal New Testament (DLNT): A Window into the Apostolic Mind The Disciples’ Literal... Read More
The Douay-Rheims 1899 American Edition (DRA): A Cornerstone of English Catholicism The Douay-Rheims ... Read More
The Easy-to-Read Version (ERV): A Bible for Everyone The Easy-to-Read Version (ERV) is a modern Engl... Read More
The English Standard Version (ESV): A Modern Classic The English Standard Version (ESV) is a contemp... Read More
The English Standard Version Anglicised (ESVUK): A British Accent on Scripture The English Standard ... Read More
The Evangelical Heritage Version (EHV): A Lutheran Perspective The Evangelical Heritage Version (EHV... Read More
The Expanded Bible (EXB): A Study Bible in Text Form The Expanded Bible (EXB) is a unique translatio... Read More
GOD'S WORD Translation (GW): A Modern Approach to Scripture The GOD'S WORD Translation (GW) is a con... Read More
The Good News Translation (GNT): A Bible for Everyone The Good News Translation (GNT), formerly know... Read More
The Holman Christian Standard Bible (HCSB): A Balance of Accuracy and Readability The Holman Christi... Read More
The International Children's Bible (ICB): A Gateway to Faith The International Children's Bible (ICB... Read More
The International Standard Version (ISV): A Modern Approach to Scripture The International Standard ... Read More
The J.B. Phillips New Testament: A Modern Classic The J.B. Phillips New Testament, often referred to... Read More
The Jubilee Bible 2000 (JUB): A Unique Approach to Translation The Jubilee Bible 2000 (JUB) is a dis... Read More
The King James Version (KJV): A Timeless Classic The King James Version (KJV), also known as the Aut... Read More
The Lexham English Bible (LEB): A Transparent Approach to Translation The Lexham English Bible (LEB)... Read More
The Living Bible (TLB): A Paraphrase for Modern Readers The Living Bible (TLB) is a unique rendering... Read More
The Modern English Version (MEV): A Contemporary Take on Tradition The Modern English Version (MEV) ... Read More
The Mounce Reverse Interlinear New Testament: A Bridge to the Greek The Mounce Reverse Interlinear N... Read More
The Names of God Bible (NOG): A Unique Approach to Scripture The Names of God Bible (NOG) is a disti... Read More
The New American Bible, Revised Edition (NABRE): A Cornerstone of English Catholicism The New Americ... Read More
The New American Standard Bible (NASB): A Cornerstone of Literal Translations The New American Stand... Read More
The New American Standard Bible 1995 (NASB1995): A Refined Classic The New American Standard Bible 1... Read More
The New Catholic Bible (NCB): A Modern Translation for a New Generation The New Catholic Bible (NCB)... Read More
The New Century Version (NCV): A Bible for Everyone The New Century Version (NCV) is an English tran... Read More
The New English Translation (NET): A Transparent Approach to Scripture The New English Translation (... Read More
The New International Reader's Version (NIRV): A Bible for Everyone The New International Reader's V... Read More
The New International Version - UK (NIVUK): A British Accent on Scripture The New International Vers... Read More
The New International Version (NIV): A Modern Classic The New International Version (NIV) is one of ... Read More
The New King James Version (NKJV): A Modern Update of a Classic The New King James Version (NKJV) is... Read More
The New Life Version (NLV): A Bible for All The New Life Version (NLV) is a unique English translati... Read More
The New Living Translation (NLT): A Modern Approach to Scripture The New Living Translation (NLT) is... Read More
The New Matthew Bible (NMB): A Reformation Revival The New Matthew Bible (NMB) is a unique project t... Read More
The New Revised Standard Version (NRSV): A Modern Classic The New Revised Standard Version (NRSV) is... Read More
The New Revised Standard Version Catholic Edition (NRSVCE): A Cornerstone of Modern Catholicism The ... Read More
The New Revised Standard Version, Anglicised (NRSVA): A British Accent on Scripture The New Revised ... Read More
The New Revised Standard Version, Anglicised Catholic Edition (NRSVACE): A Bridge Between Tradition ... Read More
The New Testament for Everyone (NTE): A Fresh Perspective The New Testament for Everyone (NTE) is a ... Read More
The Orthodox Jewish Bible (OJB): A Unique Perspective The Orthodox Jewish Bible (OJB) is a distincti... Read More
The Revised Geneva Translation (RGT): A Return to the Roots The Revised Geneva Translation (RGT) is ... Read More
The Revised Standard Version (RSV): A Cornerstone of Modern English Bibles The Revised Standard Vers... Read More
The Revised Standard Version Catholic Edition (RSVCE): A Cornerstone of English Catholicism The Revi... Read More
The Message (MSG): A Contemporary Paraphrase The Message, often abbreviated as MSG, is a contemporar... Read More
The Voice: A Fresh Perspective on Scripture The Voice is a contemporary English translation of the B... Read More
The Tree of Life Version (TLV): A Messianic Jewish Perspective The Tree of Life Version (TLV) is a u... Read More
The World English Bible (WEB): A Modern Update on a Classic The World English Bible (WEB) is a conte... Read More
The Worldwide English (WE) New Testament: A Modern Take on a Classic The Worldwide English (WE) New ... Read More
The Wycliffe Bible: A Cornerstone of English Scripture A Revolutionary Translation The Wycliffe Bibl... Read More
Young's Literal Translation (YLT): A Literal Approach to Scripture Young's Literal Translation (YLT)... Read More
For enthusiastic readers, managing a collection of books can become challenging. An expanding "to be... Read More
Deuteronomy 18 - "And if you say in your heart, 'How shall we know the word which the LORD has not ... Read More
John 14:26 - "But the Counselor, the Holy Spirit, whom the Father will send in my name, he will teac... Read More
(Enlarge) (PDF for Print) Map of the Origin of Nations and Races that were dispersed by God in Gene... Read More
The Journeys of Abraham (Enlarge) (PDF for Print) - Map of Abraham's Journey with Trade Routes Map ... Read More
(Enlarge) (PDF for Print) Map of the Route of the Hebrews from Egypt This map shows the Exodus of t... Read More
Mark 6:52 - For they considered not the miracle of the loaves: for their heart was hardened. God did... Read More
also see:The Encampment of the Children of IsraelThe Children of Israel on the March THE OUTER COURT... Read More
2 Chronicles 36:23 - Thus saith Cyrus king of Persia, All the kingdoms of the earth hath the LORD Go... Read More
All Bible Maps - Complete and growing list of Bible History Online Bible Maps. Old Testament Maps T... Read More
The Bible portrays marriage as a lifelong bond built on love, faith, and commitment, reflecting God'... Read More
Ancient Manners and Customs, Daily Life, Cultures, Bible Lands NINEVEH was the famous capital of an... Read More
Distances From Jerusalem to: Bethany - 2 milesBethlehem - 6 milesBethphage - 1 mileCaesarea - 57 m... Read More
Dagon was the god of the Philistines. This image shows that the idol was represented in the combina... Read More
Map of Israel in the Time of Jesus (Enlarge) (PDF for Print) Map of First Century Israel with Roads... Read More
The Table of Shewbread (Ex 25:23-30) It was also called the Table of the Presence. Now we will pas... Read More
see also:The PriestThe Consecration of the PriestsThe Priestly Garments The Priestly Garments 'The ... Read More
Introduction to the Book of Daniel in the Bible Daniel 6:15-16 - Then these men assembled unto the k... Read More
The Golden Lampstand was hammered from one piece of gold. Exod 25:31-40 "You shall also make a lam... Read More
The Golden Altar of Incense (Ex 30:1-10) The Golden Altar of Incense was 2 cubits tall.It was 1 cub... Read More
Ancient Tax Collector Illustration of a Tax Collector collecting taxes Tax collectors were very des... Read More
also see: Blood Atonement and The Priests The Five Levitical Offerings The Sacrifices The sacrificia... Read More
Genesis 10:32 - These are the families of the sons of Noah, after their generations, in their nation... Read More
Illustration of Jesus Reading from the Book of Isaiah This sketch contains a colored illustration o... Read More
"But the angel said unto him, Fear not, Zacharias: for thy prayer is heard; and thy wife Elisabeth s... Read More
also see: The Encampment of the Children of IsraelThe Children of Israel on the March The brazen a... Read More
In a rapidly evolving world shaped by both timeless values and groundbreaking technology, individual... Read More
Rome, the Eternal City, has stood as a symbol of history, culture, and religion for over two millenn... Read More
Many teachers assign pieces titled “an essay about god in my life.” The title invites calm thought a... Read More
Exploring identity has become part of the digital life. Social media, streaming, and gaming give you... Read More
Unearth the rich tapestry of biblical history with our extensive collection of over 1000 meticulously curated Bible Maps and Images. Enhance your understanding of scripture and embark on a journey through the lands and events of the Bible.
Start Your Journey Today!
